In the late 1800s/early 1900s, there was a desperate need for researchers to develop medications that could kill bacteria. Pneumonia and diarrhea (conditions that seem quite easy to treat today) were killing people left and right. After years of experiments and studies, manufacturers began mass-producing an antibiotic called penicillin in 1944, a medication that they called the “wonder drug”.
Antibiotics can clear a vast assortment of bacterial infections, ease symptoms, speed up the recovery process, prevent the spread of ailments from one individual to another, etc. It really is no surprise that physicians were thrilled about this medication and began widely prescribing it even if it wasn’t entirely necessary.
Dangers of the “Wonder Drug”
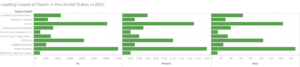
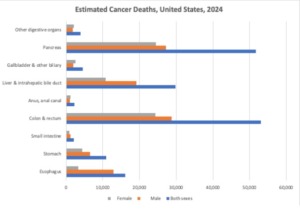
Despite all of the incredibly healing properties of antibiotics, there is also a lot of damage that they can do as well. For instance, in our bodies, the microbiome in the gut is vital to the food digestion process, breaking down toxic chemicals in the body, and assisting with the regulation of the immune system. When a person consumes antibiotics, however, the gut microbiome can be heavily disrupted, which leads to the over- or underproduction of certain chemicals that are an integral part of the immune system. This imbalance can be quite dangerous, and can actually lead to the development of various cancers.
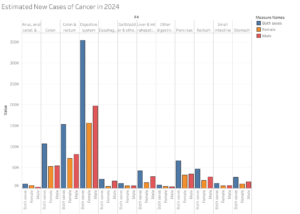
Study Linking Antibiotic Use to CRC
In 2022, researchers wanted to see if there was a specific link to antibiotic usage (particularly among younger individuals) and the development of colorectal cancer (CRC). Using census data between the years 2000 to 2011, scientists analyzed 7,903 Scottish individuals who had an official CRC diagnosis, and split them into two comparative groups:
- 445 early onset individuals (those who had received a diagnosis at under 50 years)
- 7,458 regular onset individuals (those who had received a diagnosis at over 50 years)
While comparing both groups, researchers separately analyzed antibiotic usage to see if there was a positive relationship between increased antibiotic usage and CRC development.
And what did they find?
Antibiotic usage was associated with an estimated 49% higher risk of CRC in the early onset group compared to a 9% higher risk in the regular onset group. However, a statistically-significant link was not confirmed between the two variables, meaning that a causal relationship cannot be established between antibiotic use and development of CRC.
Though researchers cannot explicitly define antibiotics as being contributors to CRC or other cancers, there is a link between the two. In terms of moving forward, more research must be conducted on this specific relationship, though researchers and physicians recommend the usual: healthy lifestyle choices to prevent CRC, regardless of antibiotic usage.
Parker Lynch is a Colorectal Cancer Prevention Intern with the Colon Cancer Foundation.